Thus ALL solutions have a water potential Ψ Less than zero 0. Discuss patterns in the direction of water movement in terms of.

Osmotic potential is one of the two components of water potential.
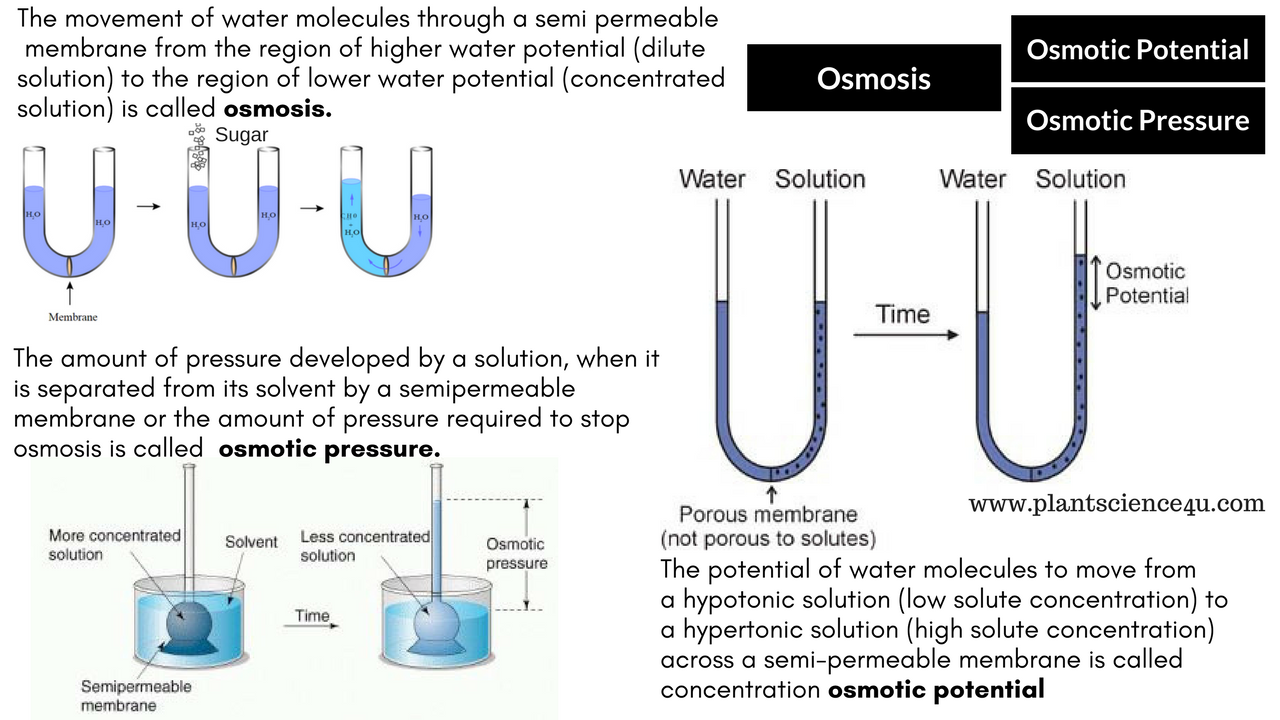
Osmosis in terms of water potential. In osmosis water diffuses across the area of lower solute concentration to that of higher solute concentration until the solute concentrations of the environment and the cell are equal. Tonicity which is the ability of a solution to gain or lose water due to osmosis results in an environment that is isotonic hypertonic or hypotonic. The water potential gradient determines the direction of osmosis.
It goes from high to low water potential. This is because n a high water potential area there is a bigger number of free water molecules. Free here means they are not attached to other solute molecules forming a hydration shell.
Osmosis is the process in which water molecules move from a region of higher water potential to a region of lower potential down a water potential gradient across a partially permeable membrane so little energy is required to carry out this process thus it is a form or passive transport. This conveys that sucrose concentration shall affect water potential therefore the higher the sucrose concentration the lower the water potential. This is how osmosis is linked to water potential and why experiments testing osmosis can be used to create methods to find andor test water potential within plants.
In addition to this concentration of sucrose in a solution and in a plant cell or plant tissue shall have an effect on water potential. Osmosis can therefore be defined as the diffusion of water from a region of high Water Potential to a region of low Water Potential through a Partially Permeable Membrane. Water Potential is measured in kiloPascals kPa where the Highest Water Potential that of pure water is 0 kPa and lower Water Potentials go into negative numbers.
Osmosis and Water Potential. In this lab you will observe the process of osmosis and diffusion. You will also learn how to calculate water potential.
If you are not familiar with these concepts make sure that you have looked them up in your textbook. If you dont know what these terms mean this lab is not going to make sense. So the net flow of water will be into the cell by osmosis from the higher water potential on the outside of the cell to the lower water potential on the inside of the cell.
This effectively causes the cell to become swollen and can sometimes cause it to burst through a process called osmotic lysis. But according to the recent trend diffusion of water is explained in terms of Water Potential. DPD is the positive value whereas water potential is the negative value.
TP is the outward pressure exerted by the cell solution on the cell wall which is developed due to osmotic diffusion of water. T temperature in Kelvins 273 degrees C pressure potential Ψp sum of all pressure in water. - an open container has 0 Ψp.
Forced caused by cell membrane pushing against cell wall. An equal and opposite force exerted by cell wall cell wall pushing back Water Potential. Water potential is the measure of the potential energy in the water while the osmotic potential is the part of the water potential that results from the presence of solute particles.
Therefore the osmotic potential is a result of dissolved solutes. Water potential Ψ is equal to pressure potential Ψp solute or osmotic potential Ψs. Osmotic potential is one of the two components of water potential.
Water potential in pure water is zero. Now that you think youve got water potential figured out lets complicate matters a little bit. Water potential Ψ is actually determined by taking into account two factors - osmotic or solute potential Ψ S and pressure potential Ψ P.
The formula for calculating water potential is Ψ Ψ S Ψ P. Osmotic potential is directly. Osmotic potential describes the dilution and binding of water by solutes that are dissolved in the water.
This potential is also always negative. Osmotic potential only affects the system if there is a semi-permeable barrier that blocks the passage of solutes. What these processes have in common is osmosis the movement of water from a region of lower solute concentration to a region of higher solute concentration.
We begin with a consideration of some basic facts about osmosis and then show how they explain. Thus ALL solutions have a water potential Ψ Less than zero 0. You cannot have a water potential Ψ great than zero 0.
Another term term you may come across when learning osmosis is osmotic pressure OP. You just need to know that the more concentrated a solution the higher the osmotic pressure. Therefore OP is the opposite of water potential Ψ meaning water will move from a low OP to a high.
Osmotic pressure is the pressure required to stop water from diffusing through a membrane by osmosis. It is determined by the concentration of the solute. Water diffuses into the area of higher concentration from the area of lower concentration.
When the concentration of the substances in the two areas in contact is different the substances. This detailed and engaging lesson describes the movement of water molecules by osmosis and this is explained in terms of water potential. Both the PowerPoint and accompanying resources have been designed to cover the third part of specification point 23 as detailed in the AQA A-level Biology specification and they also describe the impact of solutions of different water potentials on.
Water Potential Ψ Objectives 1Define osmosis in terms of water potential 2Understand water potential and its components 3Explain what happens to cells in different solutions 4Be able to calculate and predict the direction of the water movement using water potential calculations 5Apply to experimental data. Discuss patterns in the direction of water movement in terms of. Solute free water and water potential 10.
Calculate water potential given solute and pressure potential 11. Given a dialysis bagbeaker solution scenario infer which substances the dialysis tubing is.